Background: Identification of novel therapeutic targets for pharmacologic modulation of circulating von Willebrand factor (vWF) would be invaluable in treatment of von Willebrand disease, but also in other pathophysiologic processes where vWF plays a vital role. These include cardiovascular disease, cancer metastasis, angiogenesis, and smooth muscle proliferation. vWF is almost exclusively produced by the endothelial cells and stored in specialized secretory granules, Weibel Palade bodies (WPBs). We have previously shown that loss of the exocyst complex, an essential multi-subunit trafficking protein, which physically tethers secretory granules to the plasma membrane in yeast, increases vWF release. Small GTPases act as regulatable molecular switches. Cycling between GTP-bound ('on') and GDP-bound ('off') states, they control all essential cellular processes. Among these, Ral GTPase has previously been shown to be important in WPB secretion and vWF release. Since Ral specifically regulates exocyst in other cellular functions, we hypothesized that exocyst is also under direct control of Ral in vWF release.
Methods: All experiments were performed on human umbilical vein endothelial cells (HUVECs). RalA/B and exocyst depletion were performed via targeted siRNAs. vWF antigen was estimated using ELISA. RalB activation was determined by a commercial pulldown assay specific for the activated form of RalB. Expression of FLAG-tagged RalB was performed by lentiviral transduction. RalB mutants were generated using site-directed mutagenesis of FLAG-RalB (WT). Spinning disc confocal microscope was used for immunofluorescence (IF). Immuno-electron microscopy was performed using immune-gold labeling. FLAG-pulldown and immunoprecipitation (IP) was performed on cell lysates using antibody-labeled magnetic beads.
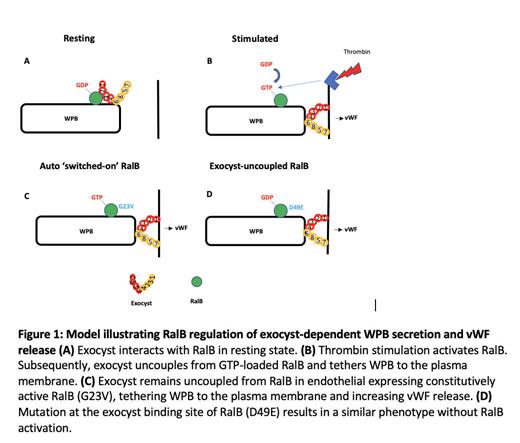
Results: RalB but not RalA depletion, or degradation with the RalB-selective inhibitor dihydroartemisinin, significantly impaired thrombin-induced vWF exocytosis (55±7% decrease). RalB was activated (GTP-bound) and phosphorylated upon thrombin stimulation of HUVECs. Correspondingly, expression of constitutively active RalB-G23V mutant, which stays in a GTP-bound state, significantly increased vWF exocytosis compared to RalB-WT (69±4.6% increase). IF and EM confirmed co-localization of RalB and exocyst on WPBs. Unexpectedly, FLAG pulldown and protein identification by mass spectrometry demonstrated that RalB-WT (GDP-bound;“off”) but not RalB-G23V (GTP-bound;“on”) interacts with exocyst, confirmed by immunoblotting. IP of resting and thrombin-stimulated native HUVEC lysates with RalB antibodies, as well as reverse-IP with exocyst antibodies confirmed this differential binding. Exocyst binding site mutation (RalB-WTD49E) and transduction of these mutants in HUVECs resulted in significantly augmented vWF exocytosis (40±3% increase), like constitutively active RalB-G23V mutant. Moreover, IF showed uniform cytoplasmic distribution of WPB cigars in RalB-WT HUVECs, whereas in RalB-WTD49E mutants WPBs were found at the plasma membrane, like RalB-G23V. This confirms that RalB-free exocyst tethers WPB to the plasma membrane ( Figure 1). Serine phosphorylation site mutation (RalB-WTS198A), previously shown to regulate GTPase activity of RalB, hence GTP-GDP cycling, also significantly decreased RalB exocyst binding, in turn increasing vWF release. This shows that post-translational phosphorylation of RalB at serine 192, which is also accelerated by thrombin activation of endothelial cells, regulates RalB-exocyst interaction, hence vWF release.
Conclusions: We have identified an important regulatory role of RalB in exocyst-dependent WPB secretion and vWF release. WPB localized exocyst is associated with RalB in resting endothelium. Endothelial cell activation switches RalB ‘on’, and exocyst, now uncoupled from GTP-bound RalB, is free to tether WPBs to the plasma membrane and facilitate vWF release.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal